Our Process
From unmet need to widespread adoption
Wherever you are in the process of developing new technology, we can help.
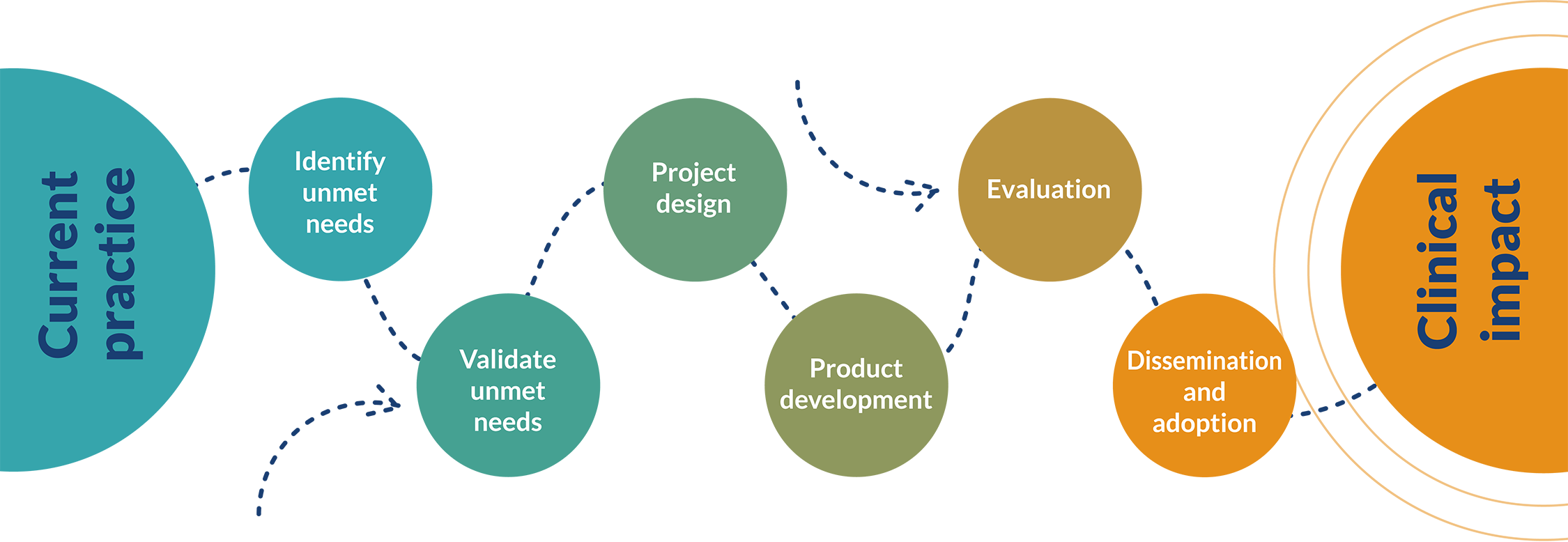
After a clinical need has been identified, we consult our networks of experts, patients, carers and communities to independently refine and validate the need. Once we know what the problem is we can start exploring creative conceptual approaches to addressing the problem, before moving towards producing and refining prototypes. This approach ensures that valuable time and resource are not wasted in developing technologies that are not fit for purpose.
Having developed prototypes, we then evaluate how they can work in clinical practice and as part of people’s daily lives. This helps us to obtain the evidence that we need in order to achieve vital CE and UKCA marks, and to provide the information needed by clinical departments and NHS purchasers to enable the device to be used in clinical practice.
When we initiate a project, we work closely with people with experience in industry to ensure that the needs of manufacturers and distributors can be anticipated, such that once we have obtained a CE mark, the device can be licensed, produced, and supplied.
We also work directly with companies that are currently developing innovative technologies that approach us with their own project concepts and devices, where Devices for Dignity are able to add value to the need validation, product development, value proposition and exploitation planning.